Dear Aspirants, Here we provide Odisha Staff Selection Commission (OSSC) Ayush Assistant Previous Year Question Paper with Answer Keys of Main Examination. The Main Examination was Held on 1st December 2019.
AYUSH (Main) – 2017:Set – 3
Composite Paper
Time: 1 hour
Full Marks: 100
Each question carries 1 mark There is negative marking of 0.25 mark for each wrong answer. Answer all questions, choosing the correct one from the alternatives suggested and darken the appropriate circle using BLUE or BLACK BALLPOINT PEN
You can read also:
1. Which of the following is wrongly matched?
(1) [Cu(NH3)4]2+ – Square planar
(2) [Nico)4] – Neutral ligand
(3) [Co(en)3]3+-follows EAN rule
(4) [Cr(NH3)6)]3+ -sp3d2
2. Flerovium is a super heavy artificial element with a symbol FI. Its atomic number is
(A 114
(B) 115
(C) 113
(D) 116
3. Acetic acid exists as a dimer in benzene due to
(A) Solvation
(B) Intermolecular H-bonding
(C) Presence of -COOH
(D) Presence of a-Haltom
4. Equal is a drug to control:
(A) Pneumonia
(B) Malaria
(C) Cold fever
(D) Mental disease
5. Which hormone contains iodine?
(A) Thyroxine
(B) Insulin
(C) Adrenaline
(D) Testosterone
6. Which of the following solution has a lower pH value?
(A) 2M NaCl(aq.)
(B) 0.2M caustic soda solution
(C) 1M NH4Cl(aq.)
(D) 1M sodium acetate solution
7. The compound which reacts faster with Lucas reagent at room temperature is;
(A) Benzyl alcohol
(B) Butan-2-ol
(C) 2-methyl propene-1-ol
(D) 2-methyl propane-2-ol
8. Which of the following process is not exothermic?
(A) Adsorption
(B) Combustion
(C) Neutralization
(D) Evaporation
9. Which of the following is a stronger conjugate base?
(A) CIO–4
(B) CIO –3
(C) CIO–2
(D) CIO–

11. A body is projected vertically upward. The times corresponding to height h while ascending and descending are t1 and t2 respectively. Then the velocity of projection is;

12. A body of mass 5 kg is thrown vertically up with K E. = 490J. The height at which the K. E. of the body becomes half of the original is
(A) 12.5 m
(B) 10 m
(C) 5 cm
(D) 500 cm
13. A child is sitting on a swing. Its minimum and maximum heights from the ground are 0.75 m and 2 m respectively. Its maximum speed will Be
(A) 10 m/s
(B) 5 m/s
(C) 8 m/s
(D) 15 m/s
14. A string vibrates with a frequency of 200 Hz. When its length is doubled and tension is altered, it begins to vibrate with a frequency of 300 Hz. Ratio of new tension to original tension is:
A) 9:1
(B) 1:9
(C) 1:34
(D) 3:1
15. A simple pendulum is suspended from the ceiling of a lift. When the lift is at rest, its time period is T. With what acceleration should the lift be accelerated upwards to reduce its period to 1/2?
(A) 2g
(B) 4g
(C) 3g
(D) g
16. Two simple harmonic motions are represented by y1 = 5[sin2pt +
√3 cos2pt] and y2 = 5 sin (211it+TT/4). The ratio of their amplitudes is:
(A) 1:3
(B) √3:1
(C) 1:1
(D) 2:1
17. A planet moving along an elliptical orbit is closest to the sun at a Distance r1 and farthest away at a distance r2 . If v1 and v2 are liner velocities at r1 and r2 then v1/v2 is
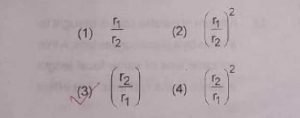
18. A body floats in water with 40% of its volume outside water. When the same body floats in an oil, 60% of its volume remains outside oil. The relative density of oil is:
(A) 0.9
(B) 10
(C) 1.2
(D) 1.5
19. Hot water cools from 60°C to 50°C in the first 10 minutes and to 42°C in the next 10 minutes. The temperature of the surrounding is:
A) 10°C
(B) 15°C
(C) 20°C
(D) 30°C
20. If a black body emits 0.5 Joules of energy per second when it is at 27°C, then the amount of energy emitted by it when it is at 627°C will be:
(A) 162J
(B) 40.5 J
(C) 135
(D) 13.5J
21. A capacitor and an inductor are connected in separate AC circuits with a bulb glowing in both circuits. The bulb glows more brightly when:
(A) A dielectric is introduced into gap between plates of a capacitor
(B) The separation between the plates of the capacitor increases the iron rod is introduced into the induction coil
(C)The iron rod is introduced into the induction coil
(D) Number of turns in the induction coil is increased
22. A current of 5 amp is flowing at 220Vin the primary coil of a transformer. If the voltage produced in the secondary coil is 2200V and 50% of power is lost, then the current in the secondary coil is
(A) 2.5 amp
(B) 0.25 amp
(C) 5 amp
(D) 0.5 amp
23. The temperature of the metal wire subjected to a constant potential difference increases, then drift velocity of the electron in it:
(A) Increases, the thermal velocity increases
(B) Decreases the thermal velocity decreases
(C) Decreases the thermal velocity increases
(D) Increases, the thermal velocity decreases
24. A charge Q is enclosed by a Gaussian sphere of radius R. If the radius is doubled, the outward electric flux will be
(A) Doubled
(B) Increased four times
(C) Reduced to half
(D) Same
25. How many 6mF, 200V condensers are needed to make a condenser of 18pF, 600V?
(A) 9
(B) 18
(C) 3
(D) 27
26. If µ0 is permeability and €0 is the permittivity of free space, then the speed of light in a vacuum is;
(1) √µ0€0

27. Two identical conducting balls has different +ve charges 9, and 9, respectively. The balls are brought together so that they touch each other and then kept in their original position. The force between them is:
(A) Greater than before the balls touched
(B) Same as that of before the balls touched
(C) Zero
(D) less than that of before the balls touched
28. A beam of parallel rays is brought toe focus by a Plano-convex lens. A thin concave lens of the same focal length is joined to the first lens. The effects:
(A)The focal point shifts towards the lens
(B)The focus remains undisturbed
(C)The focus shifts to infinity
(D)The focus shifts away from the
29. A fish in the water of refractive index n looks at a bird vertically above in the air. If Y is the height of the bird and X. is the depth of the fish from the surface, the distance of the bird estimated by the fish is:

30. A ray of light enters from rarer to denser medium. The angle of incidence is i. The reflected and refracted rays are mutually perpendicular. The critical angle is:
(A) sin-1 (tani)
(2) sin-1 (coti)
(C) tan-1(sini)
(D) cos-1(tani)
31. The spectrum of an oil flame is an example of:
(A) Line absorption spectrum
(B) Band emission spectrum
(C) Line emission spectrum
(D) Continuous emission spectrum
32. Magnetic field at the center of a circular current-carrying a coil of radius r is Bc. The magnetic field its axis at a distance r from the center is Ba
The ratio Bc/Ba is:
(A) 1: √2
(B) 1: 2√2
(C) 2√2:1
(D) √2: 1
33. There is a uniform magnetic field directed perpendicular and into the plane of the paper. An irregular shaped conducting loop is slowly changing into circular loop in the plane of the paper. Then
(A) AC is induced in the loop
(B) Current is induced in the looping anti-clockwise direction
(C) No current is induced in the loop
(D) Current is induced in the looping clockwise direction
34. An electron is moving in an orbit of hydrogen atom for which there can be maximum of six transitions. An electron moving in an orbit of another hydrogen atom for which there can be maximum of three Transitions. The ration of velocities of the electron in these two orbits:
(A) 5/4
(B) ¾
(C) ½
(D) 2/3
35. The dimensions of resistance are same as those of __ where h is Plank’s constant and e is the charge
(A) h/e2
(B) h/e
(C) h2/e2
(D) h2/e
36. A and B are two metals with threshold frequency 1.8 x 1014 Hz and 2.2 x 1014 Hz. Two identical photons of energy 0.825eV each are incident on them. Then photo electrons are emitted from
(A) A alone
(B) B alone
(C) Both
(D) Neither
37. A radioactive sample S1 having activity A1 has twice number of nuclei as another sample S2 of activity A2 IFA2 = 2A1then the ratio of half-life of S1 to that of S2, is
(1) 2
(2) 0.75
(C) 3
(D) 4
38. A radioactive element forms its own isotopes after 3 consecutive disintegration. The particles emitted are
(A) 2α-particles and 1 β-particle
(B) 2 β-particles and 1 α-particle
(C) 1 α-particle and 2 β-particles
(D) 3 β-particles
39. The ratio of velocity of sound in hydrogen and oxygen at STP is:
(A) 16:1
(B) 8:1
(C) 4:1
(D) 2:1
40. If the Sun were to increase in temperature from T to 2T and its radius from R to 2R, then the ratio of radiant energy received on earth to what it was previously will be
(A) 4
(B) 16
(D) 32
(D) 64
41. Which of the following types of light are strongly absorbed by the plants?
(A) Blue and red
(B) Indigo and yellow
(C) Orange and violet
(D) Yellow and violet
42. Who wrote Akbarnama?
(A) Dual nadir badauni
(B) Abdul Rahim Khan-e-Khanna
(C) Abu’s fazing ibn Mubarak
(D) Faizi
43. What was the form of government suggested by the cabinet’s mission plan 1946 for India?
(A) A federation
(B) A confederation
(C) A unitary from of states
(D)A union of states
44. on which of the following hills is the statio ‘Yercaud’ located?
(A)The jihadi hills
(B)The Nigari hills
(C)The phalanx hills
(D)The shevaroy hills
45. Which among the following rivers is not a tributary of the river Mahanadi?
(A)The lb.
(B)The Indravati
(C)The Ong
(D)The tell
46. Which type of farming is generally practiced in the densely populated areas of the world?
(A)Commercial farming
(B)Extensive Farming
(C)Intensive Farming
(D)Plantation Farming
47. Which of the following Union Territories has been provided with Legislative Assembly?
(A)Chandigarh
(B)Dadra and near hovel
(C)Delhi
(D)Lakshadweep
48. Which district in Odisha has the highest literacy rate as per the Census2011?
(A)Cuttack
(B) Jagatsinghpur
(C) Kendarpara
(D) Khordha
49. Which of the following trophies is game different from the other time?
(A) Durand Cup
(B) Rangaswamy Cup
(C) Santosh Trophy
(D) Rovers Cup
50. Which of the following ethnic people of the Andaman and Nicobar Islands is different from the other three on the basis of racial heritage?
(A) Jarwa
(B) Onge
(C) Sentinelese
(D) Shopmen
51. The balloon like outgrowth of parenchyma into the lumen of the vessel is known as
(A) Histogen
(B) Tyloses
(C) Phellogen
(D) Tunica
52. Vascular bundles are bicolateral in the stem of:
(A) Canna
(B) Tridua
(C) Cucurbita
(D) Possum
53. Which of the following was used by Hershey and Chase to prove that DNA is the chemical basis of heredity?
(A) TMV
(B) CMV
(C) T2 phase
(D) SPV
54. Which of the following is a bacterial disease?
(A) Influenza and Mumps
(B) Small pox and Chicken pox
(C) Polio and hydrophobia
(D) None of these
55. What is the first intermediate stable product of photosynthesis?
(A)Pep
(B)PGA
(C)PGAL
(D)Pyruvic acid
56. Camellia Saneness belongs to which family?
(A) Theaceae
(B) Musicale
(C) Loganiaceae
(D) Gamineae
57. Fungi differs from algae in
(A) Lacking chlorophyll
(B) Having cell wall of chitin and cellulose
(C) Having glycogen as reserve food material
(D) All of these
58. Which plant hormone solely responsible for fruit ripening?
(A) Auxin
(B) Abscise acid
(C) Ethylene
D) Cytokines
59. The point where the funiculi is attached to the body of ovule is called:
(A) Chalaza
(B) Hilum
(C) Ethylene
(D)Cytokinins
60. Which of the following is not a free living nitrogen fixer?
(A) Rhizobium
(B) Azotobacter
(C) Nonstoc
(D)Anabaena
61. In which form the synthesized food in plants is transported through phloem?
(A) Maltose
(B) Fructose
(C) Glucose
(D) Sucrose
62. The biological interpreter of genetic code is:
(A) t-RNA
(B) m-RNA
(C) Ribosome
(D) All of these
63. The bacterial and blue-green algae cells contain:
(A) Many linkage groups of each
(B) One linkage group of each
(C) No linkage group
(D) Only two linkage groups of each
64. Non-green large sized parenchyma with waste products is called:
(A) Mesophylls
(B) Prosennchyma
(C) Idiobiast
(D)Spongy Parenchyma
65. Which type of lichens are phycsia and parmelia?
(A) Crustose
(B) Foliose
(C) Fruticose
(D) None of these
66. Antihaemorrhagic vitamin is:
(A) Vitamin -A
(B)Vitamin – B
(C) Vitamin-E
(D) Vitamin-K
67. A hemophilic man marries a normal woman whose father was known to be a bleeder. Then it is expected that:
(A) All their children will be bleeder
(B) Half of their children will be bleeder
(C) 1/4th of their children will believe
(D) 1/3rd of their children will be bleeder
68. Self-fertilization in hydra never occurs, because they are
(A) Asexual
(B) Unisexual
(C) Protandrous
D) Protogenos
69. Lamina propria or stroma is related to:
(A) Mammalian Liver
(B) Human Intestine
(C) Pancreatic Acini
(D) Ovary of Mammal
70. Mammary glands are modifications of:
(A) Sweat Gland
(B) Sebaceous Gland
(C) Ceruminous Gland
(D) Milk Glands
71. Which of the following organ is called as ‘jack of all trades’?
(A) Kidney
(B) Pancreas
(C) Pituitary
(D) Skin
72. Ramsar Convention was held in:
(A) India
(B) Indonesia
(C) Iran
(D) German
73. Ana genesis is also known as:
(A) Phyletic Speciation
(B) Multiplicative Speciation
(C) Gradual Speciation
(D) Allopatric Speciation
74. What belongs to a class but not to family?
(A) Species
(B) Genus
(C) Order
(D) Phylum
75. Alimentary canal is not found in:
(A) Arachnida
(B) Apodaca
(C) Decapod
(D) Custody
76. Which of the following is not a component of innate immunity?
(A) Antibodies
(B) Interferon
(C) Complement protein
(D)Phagocytes
77. Which date is observed as World Animal Day?
(A) 5th June
(B) 25th July
(C) 3rd October
(D) 19th December
78. How many meiotic divisions are needed to form 100 spermatozoa?
(A) 25
(B) 50
(C) 100
(D) None of these
79. A cross between two red tomatoes produced 92 red and 31 yellow offspring’s. What are the genotypes of the parents?
(A) RR×rr
(B) Rr×rr
(C) Rr × Rr
(D) Rr×RR
80. Papillary muscles are found in:
(A) Haeckel of cockroach
(B) Auricles of heart
(C) Ventricles of heart
(D) Arm
81. Which of the following alloys generally does not contain zinc?
(A) Brass
(B) Bronze
(C) German silver
(D) Gunmetal
82. Which of the following substances contains maximum number of water molecules per mole in its crystal?
(A) Potash alum
(B) Mohr salt
(C) Washing soda
(D) Blue vitriol
83. The pure form of iron is:
(A) Pig Iron
(B) Cast Iron
(C) Wrought Iron
(3) Both absorption and adsorption
(D) Desorption
84. Which of the following pair electrodes has positive values of standard electrode potential?

Ans-B
85. Which of the following salts provides colored gas on thermal decomposition?
(A) Potassium chlorate
(B) Ammonium nitrite
(C) Sodium nitrate
(D) Lead nitrate
86. The reagent used to distinguish formaldehyde and acetaldehyde is :
(A) Alkaline iodine
(B) Alkaline phenol
(C) Tolle’s reagent
(D) Baeyer’s reagent
87. Nylon 6 is a polymer, its monomeric
(A) Hexamethylenediamine copolymer of
(B) Adeptly chloride
(C) Both (1) and (2)
(D) Caprolactam
88. The percentage of empty space in a body-centered cubic arrangement is
(A) 74
(B) 68
(C) 32
(D) 26
89. 1 mole of liquid A and 2 moles of liquid B make a solution having a total vapor pressure of 38 torr. The vapor pressure of pure A and pure Bare 45 torr and 36 torr respectively. The given solution:
(A) Is an ideal solution
(B) Shows negative deviation
(C) Is a minimum boiling azeotrope
(D) Shows positive deviation
90. When a catalyst increases the rate of a chemical reaction, the rate constant:
A) Remains constant
(B) Increases
(C) Decreases
(D) May increase or decrease depending on the order of reaction
91. The term ‘sorption’ stands for:
(A) Absorption
(B) Adsorption
(C)Both absorption and adsorption
(D)Desorption
92. Among the following electrolytes, the most effective coagulating agent for Sb2S3 solution is:
(A) K2SO4
(B) Cacl2
(C) Al2(SO4)3
(D) Na3, PO4
93. Which of the following compounds has a higher magnetic moment?
(A) [Fe(CN)6]3-
(B) [Fe(CN)6]4-
(C) [Ni(CO)4]
(D) [Fe(H2O)6]3+
94. Among the trihalides of phosphorus, __ has a higher bond angle
(A) PF3
(B) PBr3
(C) PI3
(D) PCl3
95. Which of the following is peroxoacids of sulfur?
(A) H2SO5 and H2S2O8
(B) H2SO and H2S2O7
(C) H2S2O7 and H2S2O8
(D) H2S2O6 and H2S2O7
96. In which of the following pair, the nature of hybridization is not the same?
(A) PCl3, CHCl3
(B) SO2, SnCI2
(C) NH3, NH4
(D) XeF2, BeF2
97. In the preparation of xenon compounds, Bartlett had taken Opt as a base compound. This is because:
(A) Both O2 and Xe have the same size
(B) Xenon and Oxygen are gases
(C) The oxygen molecule is paramagnetic
(D) Both dioxygen and Xenon have almost the same ΔiH values
98. In which of the following compounds, nitrogen exhibits the highest oxidation state?
(A) Hydrazine
(B) Ammonia
(C) Hydrazonic acid
(D) Hydroxylamine
99. HI can’t be prepared by action obconic. H Soon Kl because:
(A) HI is a weaker acid than H2SO4
(B) KI is an insoluble salt
(C) Both HI and H2SO4 are Oxidants
(D) HI is a strong reducing agent
100. The explosive reaction takes place when conc. H2SO4 is added to potassium permanganate. This is due to the formation of:
(A) MNO
(B) Mn2 03
(C) Mn2O5
(D) Mn2O7
You can read also:
